
Let us return to the discussion of the structure of atom. At the atomic level, the planetary model cannot explain some puzzles which arose in connection with the structure of atom. The Quantum theory has shown that the astonishing properties of atom arise from the wave-nature of the electrons, i.e. the electrons are not spherical objects but patterns of standing waves. (Standing waves can be produced on a rope, tied to a pole. When the rope is pulled tight, there is no wave, but a flick of wrist sharply downwards and then upwards creates a hump which travels down the rope to the pole, where it turns upside down, and returns to our hand. This is a travelling wave. By sending a series of humps down the rope, we can set up different patterns of standing waves. The widest point in the wave and the points at the ends of the waves remain stationary. They are called nodes. Plucking a guitar's string also produces patterns of standing waves on it.)
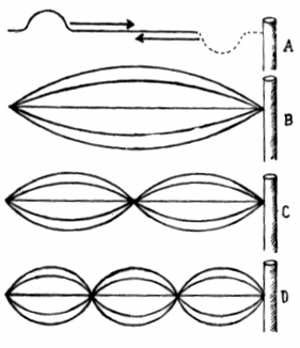
Whenever a particle is confined to a small region of space, it reacts by moving around. Smaller the region of confinement faster is the speed of the particle. Hence, while the electrons are bound to the nucleus by electric forces (which keep them as near as possible), they respond to their confinement by whirling around Tighter the bond, higher is their velocity, which is about 1000 km. per second.
Due to dual nature of the electrons, they can no longer be compared to 'planets' in the solar system. Instead they produce what is called 'electron clouds' which are made up of a various standing waves, which surround the nucleus.
Just as a fast-rotating fan appears as a disc, the high speeds of the electron-wave make the atom appear as a solid sphere. Thus, they have to settle in orbits in such a way that there is a dynamic balance between attraction of the nucleus and their tendency to escape. This means that they can rotate only in certain atomic orbits, with definite diameters. For example, the single electron of a hydrogen atom can remain in first, second or third orbit, but nowhere in-between. Normally, it will always be in its lowest orbit called 'ground state'. It can jump to a higher orbit, if receiving energy excites it. After a while, however, the electron gives off the extra energy by emitting a photon and returns back to its ground state. For all atoms with the same number of electrons, the shapes and mutual distances of their orbits are exactly the same. This is why any two atoms of hydrogen or oxygen will be totally identical. The wave nature of the electrons, thus, clarifies the puzzle of the great mechanical stability and the identity of the atoms. We can now enumerate the unusual features of the atomic level of physical reality which are not found at the macroscopic level:
- reaction with fast motion to confinement in small space
- tendencies to exist, instead of definite existence
- sudden jump from one quantum state to another
- inter-connectedness of all phenomena
On the other hand, there is nothing peculiar or special about the basic force of electric attraction between the nucleus (with + ve charge) and electron (with - ve charge), which is responsible for all atomic phenomena. It is the same old familiar force experienced at macroscopic level, between any two opposites. It is the interplay of this familiar electric charge force with the unfamiliar electron waves that is responsible for all chemical reactions and for the formation of a tremendous variety of aggregates of several atoms bound to each other by mutual attraction. Thus, the basis of the entire physical existence and the bodies of the living organisms and their biochemical processes is the interaction between the atomic nuclei and electrons. It must be remembered that in spite of the electrons constituting only a very tiny fraction of the total mass of matter in the universe, their wave-like nature gives a solid aspect to it and also provides the links necessary to build up molecular structures. They are also responsible for and are involved in the chemical reactions. On the other hand, nuclear reactions, as we shall see, generally do not take place because of extremely stable nuclear equilibrium and tremendous energies required for carrying them out.
 Jethalal S. Zaveri
Jethalal S. Zaveri
 Prof. Muni Mahendra Kumar
Prof. Muni Mahendra Kumar

